International System of Units (SI)
Units of measurement
The Metrology Act and the Order on Units of Measure stipulates that in the Republic of Slovenia units of the international system of units (SI) with the corresponding prefixes be used to express measurement results or values of physical quantities in public use. Unauthorised units may only be used in the form in parentheses after the entry of permitted units.
The SI consists of a set of basic units, prefixes and derived units. Special units outside the SI are also permitted.
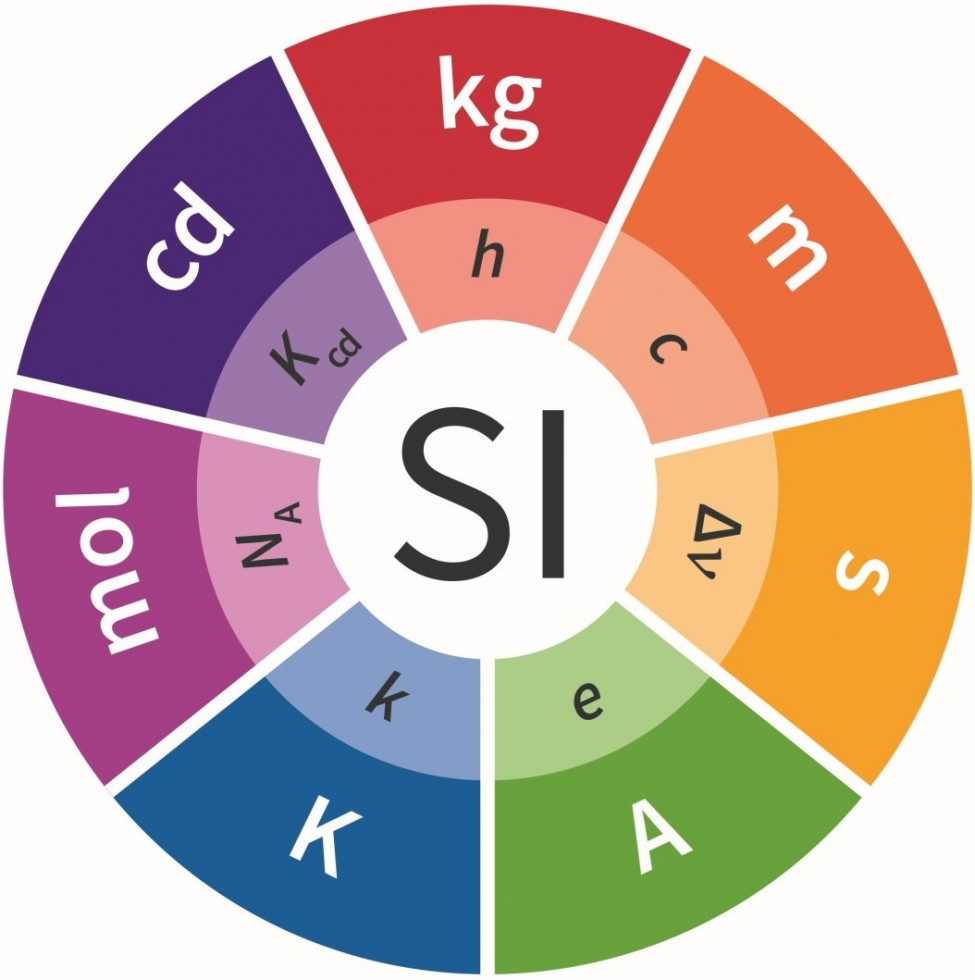
- The basic units are seven well-defined units: metre (m), kilogram (kg), second (s), ampere (A), kelvin (K), mole (mole) and candela (cd).
- Decimal multiples and divisors of SI units can be written with the SI prefixes listed in the attached table. There is no space between the prefix and the SI unit.
- Derived units are formed by combining basic units with respect to algebraic relations that connect corresponding quantities. The names and symbols of some derived units may be replaced by special names and symbols that may be used to form expressions and symbols of other derived units.
Redefining the international system of units
In 2018, the SI unit system was redefined. Since then, no unit has been determined by a materialised measure (artefact), but they are all defined through natural constants that are the same everywhere in the known universe. This defines the entire system of units (SI) in a more basic and final manner, and above all, it makes it possible to use all units anywhere and anytime.
The redefinition came into force in May 2019 and on this basis it is possible to adapt the relevant laws. The redefinition itself includes invariant determinations of seven natural constants, which lays the foundation for all other natural constants and consequently SI units. These changes were made so that the values of the newly defined SI units did not change in relation to the values that were valid until the adoption of the redefinition. This ensured a smooth transition to the new definitions, which will allow for even more accurate measurements in the future. Thus, the obstacles that could arise in the development of new technologies, materials, knowledge and further discovery of space and travel through it have been removed.
New definitions of SI units
The new definitions of SI units are as follows:
- the SI unit for mass is kilogramme with the symbol kg. It is defined by the constant numerical value of the Planck constant h, equal to 6.626 070 15 × 10-34 and expressed by the unit J s, which is equal to kg m2 s-1, where metre and second are defined by c and ΔνCs.
- the SI unit for electric current is ampere with the symbol A. It is defined by the constant numerical value of the base charge e, equal to 1.602176634 × 10-19 and expressed by the unit C, which is equal to A s where the second is defined by ΔνCs.
- the SI unit for thermodynamic temperature is kelvin with the symbol K. It is defined by the constant numerical value of the Boltzman constant k, equal to 1.380 649×10-23 J K-1, which is equal to kg m2 s-2 K-1, where kilogram, meter and second are defined by h, c and ΔνCs.
- the SI unit for the quantity of matter is mole with the symbol mol. One mole contains exactly 6.022 140 76 × 1023 elementary units. This value is the unchangeable numerical value of the Avogadro's constant NA, expressed in units of mol-1, and is called Avogadro's number. The quantity of matter of a system with the symbol n is a measure of the number of defined elementary units. The elementary unit can be an atom, a molecule, an ion, an electron or any other basic particle or a defined group of particles.
The revised definitions of the remaining three SI units are:
- the SI unit for time is second with the symbol s. It is defined by the constant numerical value of the frequency of the hyperfine transition of the cesium-133 atom in the undisturbed ground state ΔνCs, equal to 9 192 631 770 and expressed by the unit Hz, which is equal to s-1.
- the SI unit for length is metre with the symbol m. It is defined by a constant numerical value of the speed of light in vacuum c, equal to 299 792 458 and expressed by the unit m s-1, where the second is defined by ΔνCs.
- the SI unit for luminosity in a given direction is candela with the symbol cd. It is defined by the constant numerical value of the luminous efficiency of monochromatic radiation with a frequency of 540 × 1012 Hz, Kcd, equal to 683 and expressed by the unit lm W-1, which is equal to cd sr W-1 or cd sr kg-1 m-2 s3, where kilogram, metre and second are defined by h, c and ΔνCs.